Achieving New Leaps · Embarking on a New Journey
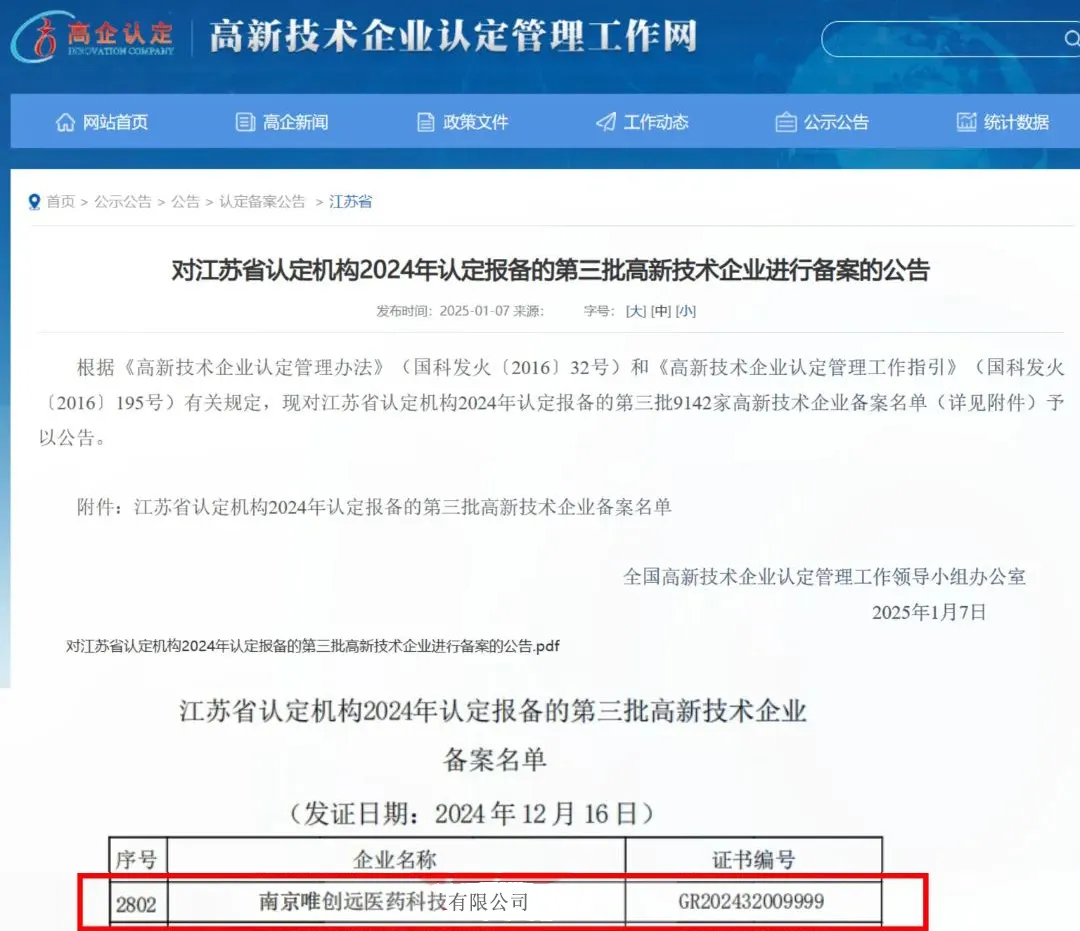
On January 7th, the Office of the National Leading Group for the Management of High-Tech Enterprise Certification, in accordance with the "Administrative Measures for the Certification of High-Tech Enterprises" (Guoke Fa Huo [2016] No. 32) and the "Guidelines for the Management of High-Tech Enterprise Certification" (Guoke Fa Huo [2016] No. 195), announced the third batch of 9,142 high-tech enterprises certified and filed by the Jiangsu Provincial Certification Authority in 2024. NanJing Vtrying Pharmatech Co., Ltd. (R&D) has successfully obtained the national "High-Tech Enterprise" certification!

NanJing Vtrying Pharmatech Co., Ltd. (R&D) successful recognition as a national "High-Tech Enterprise" marks a significant milestone in the company's development. In the future, Weichuangyuan will continue to uphold its corporate philosophy of "Only through innovation can we achieve long-term success." The company will strengthen its soft power by "attracting talent," inject vitality into its innovation capabilities through "advancing technology," and accumulate momentum for its competitiveness by "strengthening its industry presence."

NanJing Vtrying Pharmatech Co., Ltd. (R&D) is an emerging high-tech pharmaceutical company focused on the research and development of innovative drugs, improved new drugs, high-end complex formulations, and innovative medical devices, with independent intellectual property rights. The company adheres to an innovation-driven development strategy, embracing the concept of harmonizing advanced science and technology with human health and green environmental protection. By combining innovation with generic drug development and empowering the industry, NanJing Vtrying Pharmatech Co., Ltd. (R&D) focuses on clinical needs and is committed to addressing the pain points, challenges, and gaps in the industry and for its clients. The company strives to become a high-quality R&D service platform covering the entire industry chain and product lifecycle under the CPR (Chemistry + Pharmacy + Registration) model.

